First FDA Approved Cannabis-Based Drug Available for Prescription
Epidiolex, the first cannabis-based medication approved by the US Food and Drug Administration, is now available by prescription in all 50 states, according to its manufacturer GW Pharmaceuticals.
Epidiolex is intended to treat seizures associated with two rare and severe forms of epilepsy that begin in childhood called Dravet syndrome, which is a rare genetic dysfunction of the brain that begins in the first year of life, and Lennox-Gastaut syndrome, a form of epilepsy with multiple types of seizures that begins in early childhood, usually between ages 3 and 5.
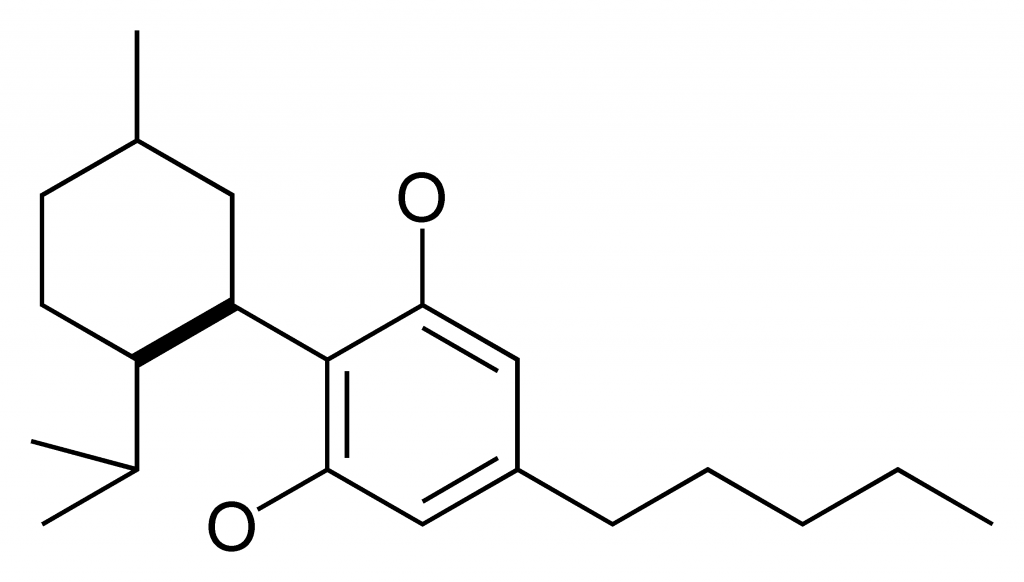
The drug is made of CBD, a component of marijuana that doesn’t give users a high and has been legal in the United States for years. According to CNN, Justin Gover, CEO of GW Pharmaceuticals, the maker of Epidiolex, said in a written statement.
“Because these patients have historically not responded well to available seizure medications, there has been a dire need for new therapies that aim to reduce the frequency and impact of seizures, “We are committed to ensuring that these patients can access this novel cannabinoid medicine that has been thoroughly studied in clinical trials, manufactured to assure quality and consistency, and is eligible to be covered by insurance for appropriate patients.”
There is much hope and speculation that the FDA will be studying the effect of CBD, especially since the uprising in states that allow medical marijuana, with more than half of The United States legalizing it.